A new study by Fondazione Michelangelo, carried out in partnership with the CRUK Cambridge Institute, shows that in triple-negative breast cancer, the cellular composition of the tumour ecosystem, especially immune cells, and their spatial distribution have a significant impact on the sensitivity or resistance to immunotherapy using immune checkpoint inhibitors (ICBs). The results of this study, published in the prestigious scientific journal Nature, could contribute to precision immuno-oncology not only in breast carcinoma but also in other cancers.
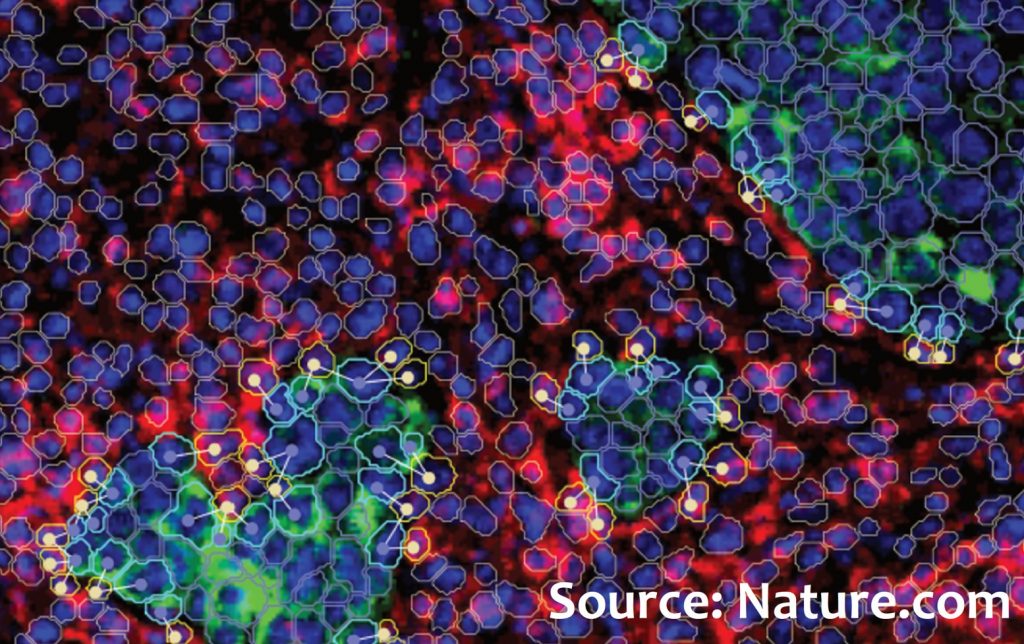
The study involved 243 patients with triple-negative breast cancer enrolled in the randomized NeoTRIP study. In this study, patients received neoadjuvant chemotherapy with or without the addition of the immune checkpoint inhibitor PD-L1, atezolizumab. Tumour samples were collected at three different time points: before therapy (n=243), after 1 month of therapy initiation (n=207), and after therapy, at surgery (n=210). Researchers used an extremely sophisticated technique called Imaging Mass Cytometry to study the determinants of the response to immunotherapy. This technique allows the simultaneous quantification of the expression of 43 key proteins at the single-cell level. Based on protein expression profiles, 17 populations of tumour/epithelial cells and 20 populations of the tumour microenvironment were identified. These populations express both cell type and functional state.
One of the most significant observations was that the presence of proliferating tumour cells expressing MHC-I and II and proliferating CD8 T cells expressing TCF1 were strongly predictive of a high likelihood of complete tumour eradication (pathological complete response, pCR), but only in the presence of immunotherapy. Utilizing information on the spatial distribution of cells, close and direct cell-to-cell contacts were quantified for each cell type. It emerged that a high number of close interactions between tumour cells and immune cells, such as B cells and CD8 T cells with high granzyme B expression (activated cytotoxic cells), were also associated with a high likelihood of benefit from immunotherapy. Furthermore, studying samples collected during treatment revealed that sensitive tumours, destined for pCR, enriched in CD8 T cells with granzyme B expression, whereas resistant tumours were characterized by an increase in the presence of CD15+ tumour cells, a potential new mechanism of adaptive resistance.
Overall, by mapping the multicellular tumour ecosystem in situ, this study demonstrated that both the proliferation level of certain cell populations and the type and quantity of cell-to-cell interactions collectively predict the response to immunotherapy in triple-negative breast cancer. The response is characterized by an influx of activated cytotoxic T cells, and the administration of ICB reshapes the tumour structure, with differences between sensitive and resistant tumours. The results of this fundamental study indicate that cellular phenotype, activation status, and spatial organization collectively contribute to the effect of atezolizumab immunotherapy. Systematic mapping of the tumour ecosystem in situ could offer a highly significant contribution to precision immuno-oncology in breast carcinoma and may also be relevant to other cancer types.
A new study by Fondazione Michelangelo, carried out in partnership with the CRUK Cambridge Institute, shows that in triple-negative breast cancer, the cellular composition of the tumour ecosystem, especially immune cells, and their spatial distribution have a significant impact on the sensitivity or resistance to immunotherapy using immune checkpoint inhibitors (ICBs). The results of this study, published in the prestigious scientific journal Nature, could contribute to precision immuno-oncology not only in breast carcinoma but also in other cancers.
The study involved 243 patients with triple-negative breast cancer enrolled in the randomized NeoTRIP study. In this study, patients received neoadjuvant chemotherapy with or without the addition of the immune checkpoint inhibitor PD-L1, atezolizumab. Tumour samples were collected at three different time points: before therapy (n=243), after 1 month of therapy initiation (n=207), and after therapy, at surgery (n=210). Researchers used an extremely sophisticated technique called Imaging Mass Cytometry to study the determinants of the response to immunotherapy. This technique allows the simultaneous quantification of the expression of 43 key proteins at the single-cell level. Based on protein expression profiles, 17 populations of tumour/epithelial cells and 20 populations of the tumour microenvironment were identified. These populations express both cell type and functional state.
One of the most significant observations was that the presence of proliferating tumour cells expressing MHC-I and II and proliferating CD8 T cells expressing TCF1 were strongly predictive of a high likelihood of complete tumour eradication (pathological complete response, pCR), but only in the presence of immunotherapy. Utilizing information on the spatial distribution of cells, close and direct cell-to-cell contacts were quantified for each cell type. It emerged that a high number of close interactions between tumour cells and immune cells, such as B cells and CD8 T cells with high granzyme B expression (activated cytotoxic cells), were also associated with a high likelihood of benefit from immunotherapy. Furthermore, studying samples collected during treatment revealed that sensitive tumours, destined for pCR, enriched in CD8 T cells with granzyme B expression, whereas resistant tumours were characterized by an increase in the presence of CD15+ tumour cells, a potential new mechanism of adaptive resistance.
Overall, by mapping the multicellular tumour ecosystem in situ, this study demonstrated that both the proliferation level of certain cell populations and the type and quantity of cell-to-cell interactions collectively predict the response to immunotherapy in triple-negative breast cancer. The response is characterized by an influx of activated cytotoxic T cells, and the administration of ICB reshapes the tumour structure, with differences between sensitive and resistant tumours. The results of this fundamental study indicate that cellular phenotype, activation status, and spatial organization collectively contribute to the effect of atezolizumab immunotherapy. Systematic mapping of the tumour ecosystem in situ could offer a highly significant contribution to precision immuno-oncology in breast carcinoma and may also be relevant to other cancer types.